To find the number of moles present in 100.0 grams of sulfur dioxide (SO2), you need to use the formula:
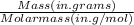
- First, you need to determine the molar mass of sulfur dioxide (SO2). Sulfur (S) has a molar mass of approximately 32.07 g/mol, and oxygen (O) has a molar mass of approximately 16.00 g/mol. Since there are two oxygen atoms in SO2, you can calculate the molar mass of SO2 as follows:
Molar mass of SO2 = (1 sulfur atom × molar mass of S) + (2 oxygen atoms × molar mass of O)
Molar mass of SO2 = (1 × 32.07 g/mol) + (2 × 16.00 g/mol)
Molar mass of SO2 = 32.07 g/mol + 32.00 g/mol
Molar mass of SO2 ≈ 64.07 g/mol
So, the molar mass of sulfur dioxide is approximately 64.07 g/mol.
- Now, you can use the formula to find the number of moles:
Number of moles = Mass (in grams) / Molar mass (in g/mol)
Number of moles = 100.0 g / 64.07 g/mol
Number of moles ≈ 1.561 moles
So, there are approximately 1.561 moles of sulfur dioxide present in 100.0 grams of sulfur dioxide.