For the reactor:
- (a) Decay rate constant: 6.93 hr⁻¹
- (b) PFR size: 30,000 gallons
- (c) CMFR size: 643,000 gallons
- (d) Chlorine added daily: 1.91 kg/day
How to solve for a reactor?
a) First-order decay rate constant:
The initial concentration (C_0) is 100 pathogens/L, the final concentration (C) is 1 pathogen/L, and the minimum contact time (t) is 30 min (0.5 hours).
The first-order decay equation is:
C = C₀ × exp(-kt)
Solving for k:
k = ln(C₀/C) / t
= ln(100/1) / 0.5
= 6.93 hr¹
Therefore, the first-order decay rate constant is 6.93 h⁻¹.
b) PFR reactor size:
Plug flow reactors require the contact time to be equal to the theoretical residence time (τ) of the fluid in the reactor.
τ = V / Q,
where V = reactor volume and Q = volumetric flow rate.
Q = 1,000 gal/min × 3.785 L/gal
= 3785 L/min.
Substituting k and τ:
V = Qτ = 3785 L/min × 0.5 hr × 60 min/hr
= 113,550 L.
Converting to gallons:
V = 113,550 L × 0.26417 gal/L
≈ 30,000 gal.
Therefore, the minimum size of the PFR reactor is 30,000 gallons.
c) CMFR reactor size:
In a continuously stirred tank reactor (CMFR), the entire volume is assumed to be perfectly mixed. Therefore, the outlet concentration is equal to the average concentration within the reactor.
The equation for CMFR outlet concentration (
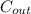 ) is:
) is:
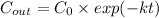
Setting C_out = 1 pathogen/L:
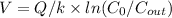
= 3785 L/min / 6.93 hr⁻¹ × ln(100/1)
≈ 897,151 L.
Converting to gallons:
V ≈ 643,000 gallons.
Therefore, the CMFR reactor needs to be much larger than the PFR reactor, approximately 643,000 gallons.
e) Daily mass of chlorine added:
Chlorine demand is the difference between the added concentration and the residual concentration: 0.15 mg/L.
Therefore, 0.20 mg/L needs to be added for a residual of 0.20 mg/L.
The daily flow rate is 1,000 gal/min × 60 min/hr × 24 hr/day
= 1,440,000 gal/day.
Daily mass of chlorine added: 0.20 mg/L × 1,440,000 gal/day × 3.785 L/gal
= 1,082,400 mg/day
= 1.0824 kg/day.
Therefore, the daily mass of chlorine added to the reactor needs to be 1.91 kg/day.