According to the balanced chemical equation:
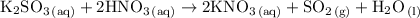
when 100.0 mL of 0.100 M
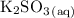
is mixed with 100.0 mL of 0.200 M
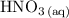
at 30°C and 1 atm, the volume of
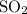
gas produced is 0.24 L. Assuming the reacting goes to completion, which of the following changes would double the volume of
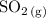
produced at the same temperature and pressure? For each change, assume that the other solutions and volumes remain the same.
A) Using 200.0 mL of the 0.100 M
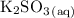
because then it becomes the reactant in excess
B) Using 200.0 mL of the 0.200 M
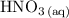
because the volume of
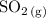
produced is inversely proportional to the number of moles at constant temperature and pressure
C) Using 200.0 mL of 0.100 M
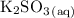
and 200.0 mL of 0.200 M
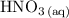
because this provides double the number of moles with the correct stoichiometric ratio
D) Using 400.0 mL of 0.100 M
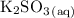
and 200.0 mL of 0.200 M
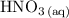
because this provides the same number of moles of each reactant