Answer:
The density measurement would be smaller than the actual value.
Step-by-step explanation:
The density of an object can be found by dividing the mass of the object by its volume:
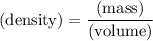 .
.
Assume that the volume of this metal object is found in the following steps:
- Add water to a volumetric cylinder.
- Record initial reading of the volume.
- Immerse the metal object in the water in the cylinder.
- Record the new volume reading.
If there is no air bubble, the volume of the metal object would be equal to the volume of water displaced. That volume is supposed to be equal to the difference between the two volume readings.
However, in this example, the air bubbles attached to the metal object would displace more water than the metal object alone could displace. The volume difference would be an overestimate of the actual volume of the metal object.
Assuming that the mass of the metal object is measured correctly. In the expression for density, mass (numerator of the fraction) would stay the same while volume (the denominator) would be larger than the actual value. The density estimate, which is equal to the value of this fraction, would be smaller than the actual value.