The wavelength of light with a frequency of
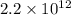 Hz is approximately 136.36 micrometers.
Hz is approximately 136.36 micrometers.
To calculate the wavelength of light with a frequency of
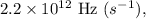 we use the relationship between wavelength
we use the relationship between wavelength
 frequency (f), and the speed of light (c). The speed of light in a vacuum is a constant,
frequency (f), and the speed of light (c). The speed of light in a vacuum is a constant,
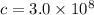 meters per second (m/s).
meters per second (m/s).
The formula to relate these quantities is:
![\[ c = \lambda * f \]](https://img.qammunity.org/2024/formulas/physics/high-school/le431274f7w4njwul6vq3aa9gzu7ac4qy4.png)
Where:
- c is the speed of light (about
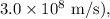
-
 is the wavelength (which we are trying to find),
is the wavelength (which we are trying to find),
- f is the frequency
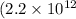 Hz in this case).
Hz in this case).
Rearranging this formula to solve for the wavelength
 , we get:
, we get:
![\[ \lambda = (c)/(f) \]](https://img.qammunity.org/2024/formulas/physics/college/z9u9l83m3b78cmom56shcf178fupswix3y.png)
Let's calculate the wavelength using this formula.
The wavelength of light with a frequency of
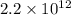 Hz is approximately 0.000136 meters, or 136.36 micrometers.
Hz is approximately 0.000136 meters, or 136.36 micrometers.