The number of vacancies at 646°C is approximately 1.28 x 10²⁴ m³.
How to calculate the number of vacancies?
Convert temperatures to Kelvin:
T₁ = 879°C + 273.15
T₁ = 1152.15 K
T₂ = 646°C + 273.15
T₂ = 919.15 K
Calculate the change in free energy:
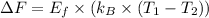
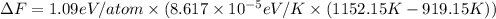
ΔF ≈ 0.63 eV/atom
Calculate the ratio of vacancy concentrations:
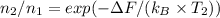
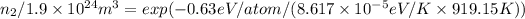
 ≈ 1.28 x 10²⁴ m³
≈ 1.28 x 10²⁴ m³
Therefore, the number of vacancies at 646°C is approximately 1.28 x 10²⁴ m³.
Complete question:
The number of vacancies in some metal at 879 C is 1.9 x 10²⁴ m³. Calculate the number of vacancies at 646 C given that the energy of vacancy formation is 1.09 ev/atom ; assume the density at both temperatures is the same.