Answer: A) 127.1 amu
Step-by-step explanation:
To find the average atomic mass of an element, we multiply each isotope by its abundance, add them all together, then divide by 100.
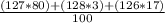 = 126.9 amu
= 126.9 amu
The closest choice to my answer is A) 127.1 amu, which makes sense because iodine-127 has the highest abundance at 80%, and the isotope with the highest abundance is always the closest to the average atomic mass of the element.
I hope this helps! :)