Answer: 1793.5g LiCl
Step-by-step explanation:
This is a unit conversion problem, and we will be converting from mol LiCl to g LiCl using our given value (42.30 mol LiCl) and the molecular mass of LiCl (42.40g LiCl/1 mol LiCl).
I wrote it two different ways in case the fraction is a bit difficult to read. :)
42.30 mol LiCl (
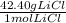 ) = 1793.5g LiCl
) = 1793.5g LiCl
42.30 mol LiCl (42.40g LiCl/1 mol LiCl) = 1793.5g LiCl
If it's asking for significant figures, I would put 1794g LiCl, because our given value has 4 significant figures, so we would have the same number of significant figures in our final answer. The exact answer was 1793.52, so we round up to 1794.
I hope this helps! :)