Answer:
Approximately
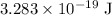 ;
;
Approximately
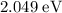 .
.
(Assumption: the wavelength was measured in vacuum, where the speed of light is
 .)
.)
Step-by-step explanation:
The per-photon energy of this light can be found from its wavelength through the following steps:
- Find the frequency of this light from its wavelength.
- Find the per-photon energy of this light using the Planck Energy-Frequency Relation.
It is given that the wavelength of this light is
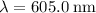 . In standard units (meters,) the wavelength of this light would be:
. In standard units (meters,) the wavelength of this light would be:
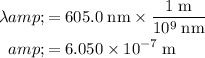 .
.
To find the frequency
 of this light, divide the wave speed
of this light, divide the wave speed
 by the wavelength:
by the wavelength:
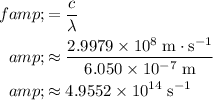 .
.
By the Planck Energy-Frequency Relation, if the frequency of an electromagnetic wave is
 , the per-photon energy
, the per-photon energy
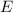 of this wave would be:
of this wave would be:
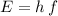 ,
,
Where
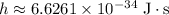 is the Planck Constant.
is the Planck Constant.
Substitute in
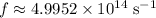 and evaluate to find the per-photon energy of this wave:
and evaluate to find the per-photon energy of this wave:
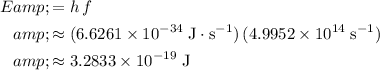 .
.
Given that
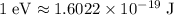 , this per-photon energy would be equivalent to:
, this per-photon energy would be equivalent to:
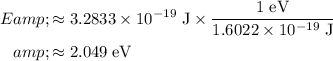 .
.