Answer:
72%
Step-by-step explanation:
Percent yield is the amount a reaction yields compared to what the reaction is expected to yield.
Defining Percent Yield
In every reaction, we can calculate how much the reaction should produce using stoichiometry. The closer the yield is to 100%, the more successful the reaction was. If the percent yield is too low, then we know that there was an error in the lab or that one of the samples used in the experiment was impure. Additionally, the percent yield cannot be over 100% due to the law of conservation of mass. If the calculated percent yield was over 100%, then we know that there was an error in the experiment as well.
Calculating Percent Yield
Percent yield is calculated using a formula. The percent yield formula is as follows:
In this reaction, the expected yield is 85.3g and the actual yield is 61g. So, we can plug these values into the formula.
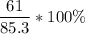 = 72%
= 72%
Remember to round to significant figures (sig figs) for percent yield. Since the actual yield has 2 sig figs, so should the percent yield. The percent yield for the reaction is 72%. This shows that there was likely some form of error in the experiment because the percent yield is notably lower than 100%.