Answer: 4.11 g
Step-by-step explanation:
First off, we want to determine the amount of KCIO₃that is being used in the reaction. To find this, we can use the molar mass of KCIO₃. The molar mass of KCIO₃ can be found by adding up the molar masses of each atom present in the compound, of which can be found on a periodic table.
So, the molar mass of KCIO₃ is equal to
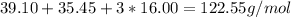
Notice that we multiply the molar mass of oxygen by 3, as there are 3 oxygen atoms in the compound.
This means that every mole of KCIO₃ is 122.55 grams.
To find the moles of KCIO₃ from here, we just need to divide the grams by the molar mass to find how many moles are in the given mass of KCIO₃.
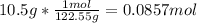
Now that we have the moles of KCIO₃, 0.0857 moles, we can set a ratio of the coefficients of KCIO₃ and the product we want to determine the moles of, O₂.
According to the coefficients of KCIO₃ and O₂, we can see that every 2 moles of KCIO₃ used up produces 3 moles of O₂, so the ratio is
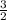 .
.
To find the moles of O₂, we just then need to multiply the ratio we just obtained by the number of moles of KCIO₃.
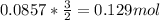
So, the reaction produces 0.129 moles of O₂. Almost there.
The question asks for the mass of O₂. We just need to convert moles to grams. We already know how to convert grams to moles, and moles to grams is very similar.
We again need the molar mass, but for O₂ this time. There are 2 oxygens in O₂ as shown by the subscript 2, so the molar mass of O₂ will be twice the molar mass of oxygen. So, the molar mass of O₂ is 32.0 g/mol. Every mole of O₂ is 32.0 grams.
Take this molar mass and multiply by the number of moles to find grams.
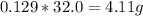 .
.
This reaction produces 4.11 grams of O.
Once you know the basics of a stoichiometry problem, you can do it in one step. That would look like:
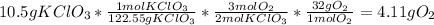
In here you can see me do every step I showed above, just condensed into one equation.