Answer:
50 moles of Oxygen gas would be required to completely react with 4 moles of octane.
Step-by-step explanation:
Side note: Throughout this explanation, we'll be referring to oxygen atoms (just a single O), and oxygen gas (
 ). Hopefully the usage is clear from context, but I'll try to be explicit.
). Hopefully the usage is clear from context, but I'll try to be explicit.
Main concepts
Concept 1. Alkanes
Concept 2. Combustion reactions
Concept 3. Stoichiometry
Concept 1. Alkanes
Several chemical compounds fall into a class of compounds called "alkanes". Alkanes are a type of molecule which consist of a chain of single-bonded carbons, and hydrogen atoms that fill out the balance of the necessary bonds for each carbon.
Each carbon wants 4 bonds, so carbons on the end of the chain will only be bonded to 1 carbon, and will need 3 more bonds -- 3 hydrogens. In contrast, each carbon in the middle of the chain will already have a carbon bonded on either side of it, so it will already have 2 bonds, and will only need 2 more bonds -- 2 hydrogens.
A general formula for the number of carbons and hydrogens is given by
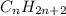 where "n" is the number of carbons in the chain (the number of hydrogens will be two more than double the number of carbons).
where "n" is the number of carbons in the chain (the number of hydrogens will be two more than double the number of carbons).
Alkanes are named with prefixes based on the number of carbons in the chain, followed by the suffix -"ane". Prefixes for the first 10 numbers are listed in the table below:

So, in this case, Octane means that the alkane has 8 carbons.
This means the general formula for Octane is
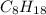
Concept 2. Combustion reactions
All combustion reactions require oxygen gas.
For combustion reactions of substances containing only Carbon, Oxygen, and/or Hydrogen, the products of the reaction are Carbon dioxide
 and Water
and Water

Since Octane only contains Carbon and Hydrogen, the reaction will produce Carbon dioxide and water.
Unbalanced chemical reaction:
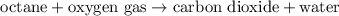

To balance the equation,
- observe that there are 8 carbon atoms on the left side of the reaction. Increase the coefficient of the Carbon dioxide to 8 to ensure there are 8 carbons on the right.
- observe that there are 18 hydrogen atoms on the left side of the reaction. Increase the coefficient of the water to 9 to ensure there are 18 hydrogen atoms on the right.
semi-balanced chemical equation
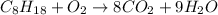
Now, by increasing the coefficients of the Carbon dixoide and water to balance the Carbons and the Hydrogens, the number of oxygen atoms on the right has changed. In total, on the right, there are 25 oxygen -- 16 oxygens from the eight carbon dioxides, and 9 oxygens from the nine waters.
To get 25 oxygen atoms on the left, we'll need to increase the number of Oxygen gas molecules on the left to 12.5.
However, it is common to use only whole numbers as coefficients, so to rid ourselves of the decimal, we'll need to multiply all of the coefficients by 2 (the least common denominator of all of the coefficients).
Final Balanced chemical equation

Concept 3. Stoichiometry
If we start with 4 moles of octane, we'll need the mole ratio from the balanced chemical equation to find the number of moles of oxygen gas.
The final balanced chemical reaction requires 2 moles of octane with 25 moles of oxygen gas.
So
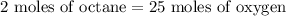
Creating a unit ratio with moles of octane on the bottom, we'll divide both sides of that equation by the 2 moles of octane.

Observe that the units on the left completely cancel, and 2/2 is 1...
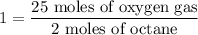
So, we have obtained a unit ratio
Using this unit ratio to the 4 moles of octane, we obtain the result of moles of oxygen gas:
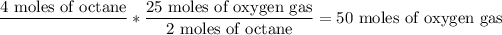
So, 50 moles of Oxygen gas would be required to completely react with 4 moles of octane.