Key Concepts
- Mole ratios
- Limiting reagent
- Balancing chemical equations
Solution
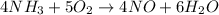
We're given:
The mole ratio between NH3 and O2 is 4:5, meaning...
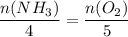
Plug in the given moles of NH3 and solve for moles of O2:
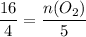
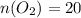
Answer
20 moles of O2 are needed to completely react with 16 moles of NH3.