Step-by-step explanation:
First you write the balanced chemical equation
- 2 C2H6 + 7O2 > 4CO2 + 6H2O
Then you put X on the above of water and put 35 L on the above of O2.
35 L X
2. 2 C2H6 + 7O2 > 4CO2 + 6H2O
After that you multiply the coefficient of O2 by STD( 22.4 L ) and also H2O. And write the number on below O2 and H2O.
35 L X
3. 2 C2H6 + 7O2 > 4CO2 + 6H2O
156.7 L 134.4 L
4. Then make a proportion
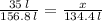
X = 30 L
next change the volume to mole
5. 1 mol = 22.4 L
X = 30 L
X ~ 1.34
Finally change the mole to mass by the formula of:
mass of water = mole of water × molar mass of water
m = m × M
m = 1.34 mol × 18 g\mol
m = 24.12 g