Answer:
5.54mL = V2
Step-by-step explanation:
V1 = 11.5mL
T1 = 415k
T2 = 200k
V2 = ?
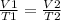 so this means
so this means
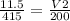 then you cross multiply
then you cross multiply
(11.5)(200) = (415)(V2)
2300 = 415 x V2
then you have to divide
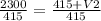
then you cross out the 415 from the top and bottom of second part so its not there so it would be
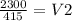
then divide
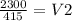 so V2 = 5.54mL (mL bc thats the only volume in the equation)
so V2 = 5.54mL (mL bc thats the only volume in the equation)