A two-phase liquid–vapor mixture with equal volumes of saturated liquid and saturated vapor has a quality of 0.5: False.
In Science, a mixture can be defined as a combination of two (2) or more substances which are present in varied proportion (unfixed ratios). Additionally, a mixture can be visibly seen with our na-ked eyes and separated by physical means.
Generally speaking, the ratio of the mass of vapor to the total mass of the miaxture for a two-phase liquid-vapor mixture is typically referred to as its quality and denoted by the letter x. Mathematically, this can be modeled as follows;
Quality, x =
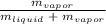
At saturated liquid and saturated vapor states, the value of x are equal to 0 and 1.0 resepctively. In this context, a vapor with the same volume as the liquid would have a smaller mass, which means x is less than 50%.