Answer:
1M
Step-by-step explanation:
To find molarity, we use the formula
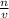 , where n is the moles of solute, and v is the volume in liters.
, where n is the moles of solute, and v is the volume in liters.
This formula is fairly straightforward. When we input our values we get a formula of
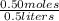 . Simplifying yields 1M. (Hint: Don't forget that occasionally we do have to convert between units. In this case, the 500mL was converted to liters using the known conversion factor that 1000mL= 1 liter.)
. Simplifying yields 1M. (Hint: Don't forget that occasionally we do have to convert between units. In this case, the 500mL was converted to liters using the known conversion factor that 1000mL= 1 liter.)