Answer:
2.69 L
Step-by-step explanation:
Volume is directly proportional to temperature, so we can use the following formula:
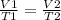
V₁ = 2.50 L
T₁ = 298 K
T₂ = 321 K
If we plugin the following values we end up with the equation:
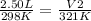
If we solve for V₂ using a calculator we end up with V₂ ≈ 2.69 L