For
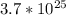 molecules of
molecules of
 , there are approximately 61.56 moles of
, there are approximately 61.56 moles of
 based on Avogadro's number, which relates the number of entities to moles.
based on Avogadro's number, which relates the number of entities to moles.
To determine the number of moles in a given number of molecules, you can use Avogadro's number, which defines the number of entities (atoms, molecules, etc.) in one mole. Avogadro's number is approximately
 entities/mol.
entities/mol.
In this case, you have
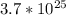 molecules of
molecules of
 . To find the number of moles, use the formula:
. To find the number of moles, use the formula:
![\[ \text{Number of moles} = \frac{\text{Number of molecules}}{\text{Avogadro's number}}. \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/6gap96x9gfvvdjp9olrrtx103eq9yjyrhf.png)
Substitute the values:
![\[ \text{Number of moles} = (3.7 * 10^(25))/(6.022 * 10^(23)). \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/2lcd2re8nmo416rxh0ox547vvic88str1h.png)
Calculating this yields the number of moles of
 molecules.
molecules.
![\[ \text{Number of moles} \approx 61.56 \text{ moles of } F_2. \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/w0js6n3k3pbhw2ohv8s1bph0zo16b3w6w4.png)