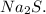Final answer:
If you have 18.3 moles of
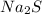 , you would start with 1007.398 grams of
, you would start with 1007.398 grams of
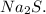
Step-by-step explanation:
To determine the mass of
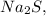 we need to know the molar mass of
we need to know the molar mass of
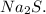 Sodium (Na) has a molar mass of 22.99 g/mol and sulfur (S) has a molar mass of 32.07 g/mol. Adding them together, the molar mass of
Sodium (Na) has a molar mass of 22.99 g/mol and sulfur (S) has a molar mass of 32.07 g/mol. Adding them together, the molar mass of
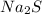 is 55.06 g/mol.
is 55.06 g/mol.
Given that you have 18.3 moles of
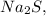 you can calculate the mass by multiplying the number of moles by the molar mass:
you can calculate the mass by multiplying the number of moles by the molar mass:
Mass = Moles x Molar mass
Mass = 18.3 moles x 55.06 g/mol
Mass = 1007.398 g
Therefore, you would start with 1007.398 grams of