Answer:
Synthesis.
Step-by-step explanation:
Hello!
In this case, since we can find synthesis, decomposition and single and double displacement reactions, it is possible for us to realize this reaction is synthesis because sulfur and fluorine react to produce sulfur hexafluoride according to:
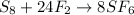
These reactions are characterized by the presence of two or more reactants and just one product.
Best regards!