Answer:
rₙ = 1,325 10⁻⁹ m
Step-by-step explanation:
To solve this problem we use the bohr atomic model
Eₙ = -13.606 /n² [eV]
the brackets indicate that the units are in electron volts.
let's reduce the photon energy to eV
E = 4.5 10-19J (1 eV / 1.6 10⁻¹⁹ eV) = 2.8125 eV
This energy is in the visible range, so the transition must occur in this range, this is for the Balmer series whose initial number is n₀ = 2
for an atomic transition on two levels
ΔE = Eₙ - E₀ =
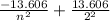
2.8125 =
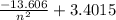
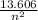 = 3.4015 - 2.8125 = 0.589
= 3.4015 - 2.8125 = 0.589
n² = 13.606 / 0.589
n² = 23.1
n = 4.8
as n must be an integer
n = 5
taking the quantum number as far as the electron goes, we substitute in the equation for the radius
rn = n² a₀
where ao is the radius of the lowest level a₀ = 5.3 10⁻¹¹ m
rₙ = 5 2 5.3 10⁻¹¹
rₙ = 132.5 10⁻¹¹ m
rₙ = 1,325 10⁻⁹ m