Step-by-step explanation:
The given chemical equation is as follows.

In order to balance the equation, the number of reactants must be equal to the number of products. So, when four moles of sodium reacts then the chemical equation will be as follows.
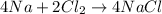
Thus, we can conclude that when four moles of sodium reacts then two moles of chlorine gas will react.