Molar mass Zinc chloride
ZnCl2 = 65.3 + 35.5 * 2 = 136.3 g/moles
1 mole ZnCl2 ------------- 136.3 g
x mole ZnCl2 ------------- 302.48 g
x = 302.48 / 136.3
x = 2.219 moles
1 mole --------------- 6.02x10²³ units
2.219 moles --------- y units
y = 2.219 * 6.02x10²³
y =
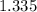
formula units
hope this helps!.