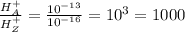Answer: (4) 1000
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/9p2aoktrfyrswsdfh3xbgetuhore5bd1n7.png)
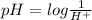
Thus as pH and
 are inversely related, a solution having lower pH will have more amount of
are inversely related, a solution having lower pH will have more amount of
 concentration. And a solution having more pH will have less amount of
concentration. And a solution having more pH will have less amount of
 concentration.
concentration.
1. Solution A has a pH of 3
![13=-log[H^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/828sa2qitief314chl5jqcfiwm8u2wauyn.png)
![[H^+_A]=10^(-13)](https://img.qammunity.org/2017/formulas/chemistry/high-school/bxpueatz7piszztoodf7mn09p3kq5wv9qx.png)
2. solution Z has a pH of 6.
![16=-log[H^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/svyo74jxbauppw8ztgq5wajtuc6wyaqfj7.png)
![[H^+_Z]=10^(-16)](https://img.qammunity.org/2017/formulas/chemistry/high-school/ktdgczj5ltfulbg8y7a722054391d6jbzn.png)
Thus Solution A with low pH has higher
 concentration.
concentration.