Answer:1.4 moles of calcium chloride are required.
Step-by-step explanation:
Molarity of the calcium chloride solution = 0.700 M = 0.700 mol/L
Volume of the solution = 2 L
Moles of the calcium chloride = x
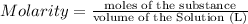
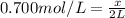
x = 1.4 moles of calcium chloride
1.4 moles of calcium chloride are required.