Answer;3.67061 kg of gold must be solidified to release 235 kJ of energy.
Step-by-step explanation:
Molar heat of gold = 12.550 kJ/mol
1 mole of gold releases = 12.550 kJ/mol
Or we can rewrite it as ,1 kJ of energy is given by
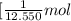
Then 235 kJ of energy will be released by:
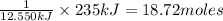 of gold
of gold
Mass of gold in 18.72 moles = 18.72 mole × 196.08 g/mol =3,670.61 g =3.67061 kg
1 kg = 1000 g
3.67061 kg of gold must be solidified to release 235 kJ of energy.