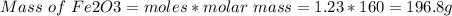Answer:
Amount of Fe2O3 produced is 197 g
Step-by-step explanation:
Step 1: Deduce the limiting reactant
Mass of FeS2 = 294 g
Molar Mass of FeS2 = 120 g/mol
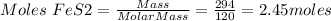
Mass of O2 = 176 g
Molar mass of O2 = 32 g/mol
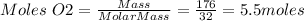
Since moles of FeS2 < O2, then FeS2 is the limiting reactant which will dictate the amount of product formed
Step 2: Calculate the mass of Fe2O3 produced
The balanced equation is:
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
Based on the reaction stoichiometry:
4 moles of FeS2 produced 2 moles of Fe2O3
Therefore, 2.45 moles of FeS2 will produce 1.23 moles Fe2O3
Molar mass of Fe2O3 = 160 g/mol