Answer: Number of half lives occurred 2.
Step-by-step explanation:
Let the initial concentration be x
Concentration left=25 % of the x=

To calculate the number of half lives, we use the formula:
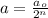 ..(1)
..(1)
where,
a = amount of reactant left after n-half lives = 0.0495 moles
 = Initial amount of the reactant = 0.7924 moles
= Initial amount of the reactant = 0.7924 moles
n = number of half lives

taking log both sides



n = 2
Number of half lives occurred 2.