Answer:
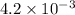 M
M
Step-by-step explanation: The equilibrium reaction for dissociation of weak acid is,
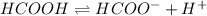
initially conc. c 0 0
At eqm.
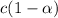
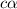
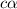
The expression for dissociation constant is:
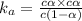
when
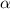 is very very small the, the expression will be,
is very very small the, the expression will be,
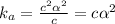
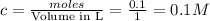
Now put all the given values in this expression, we get
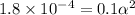
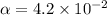
![[H^+]=c\alpha=0.1\\times 4.2* 10^(-2)=4.2* 10^(-3)M](https://img.qammunity.org/2017/formulas/chemistry/high-school/317xhkvq17sm942cl7tztxm27it57n8o7v.png)