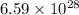Answer: The number of photons absorbed by the water is
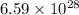
Step-by-step explanation:
- To calculate the energy of 1 photon, we use the equation:

where,
E = energy of the photon
h = Planck's constant =
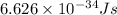
c = speed of light =
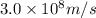
 = wavelength of photon = 13.3 cm = 0.133 m (Conversion factor: 1 m = 100 cm )
= wavelength of photon = 13.3 cm = 0.133 m (Conversion factor: 1 m = 100 cm )
Putting values in above equation, we get:
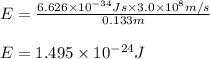
To calculate the mass of water, we use the equation:

Density of water = 1 g/mL
Volume of water = 0.317 L = 317 mL (Conversion factor: 1 L = 1000 mL )
Putting values in above equation, we get:
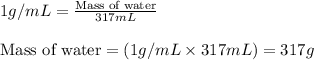
- To calculate the amount of energy absorbed, we use the equation:

where,
q = heat absorbed
m = mass of water = 317 g
c = specific heat capacity of water = 4.184 J/g°C
 = change in temperature = 74.3°C
= change in temperature = 74.3°C
Putting values in above equation, we get:
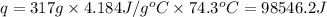
- To calculate the number of photons, we divide the amount of energy absorbed by the energy of 1 photon, which is:
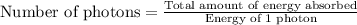
Putting values in above equation, we get:
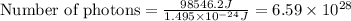
Hence, the number of photons absorbed by the water is