Answer: The correct option for the chemical equation
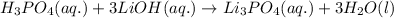 is 1.
is 1.
The correct option for the chemical equation
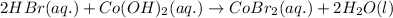 is 3.
is 3.
Step-by-step explanation:
- For the chemical equation:
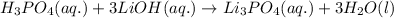
 is a weak acid and hence will not dissociate into ions whereas LiOH is a strong base and will easily dissociate into ions.
is a weak acid and hence will not dissociate into ions whereas LiOH is a strong base and will easily dissociate into ions.
The product
 is soluble in water and hence, will dissociate into its respective ions. Hence, the ionic equation for this reaction is:
is soluble in water and hence, will dissociate into its respective ions. Hence, the ionic equation for this reaction is:
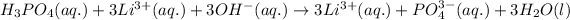
Net ionic equation becomes:
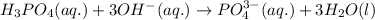
So, the correct option is 1.
- For the chemical equation:
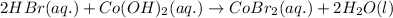
HBr and
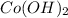 are strong acid and strong base respectively, hence they will easily dissociate into ions.
are strong acid and strong base respectively, hence they will easily dissociate into ions.
The product,
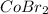 is is soluble in water and hence, will dissociate into its respective ions. Hence, the ionic equation for this reaction is:
is is soluble in water and hence, will dissociate into its respective ions. Hence, the ionic equation for this reaction is:
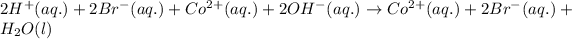
The net ionic equation becomes:

So, the correct option is 3.