Answer: Helium is least likely to form bonds.
Step-by-step explanation:
Helium has the electronic configuration
 . As the 1s sub-shell is completely filled so there is no need for helium to form bonds with other atoms.
. As the 1s sub-shell is completely filled so there is no need for helium to form bonds with other atoms.
Whereas, nitrogen has the electronic configuration
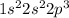 . As the p sub-shell is half filled, it has the tendency to accept more electrons. Therefore, nitrogen is more susceptible to form bonds as compared to helium.
. As the p sub-shell is half filled, it has the tendency to accept more electrons. Therefore, nitrogen is more susceptible to form bonds as compared to helium.
Thus, it is concluded that helium is least likely to form bonds.