Answer: The volume of mercury spilled is 0.61 ml.
Step-by-step explanation:
Density is defined as the mass contained per unit volume.

Given : Mass of mercury= 8.3 grams
Density of mercury=
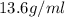
Putting in the values we get:
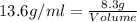
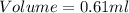
Thus the volume of mercury spilled is 0.61 ml.