Answer: Option (B) is the correct answer.
Step-by-step explanation:
In a metallic bond, there occurs a sea of electrons which is uniformly distributed throughout the solid substance or material.
Metallic bonds always form between metals.
In a ionic bond, there will always be transfer of electrons from one atom to another.
An ionic bond is always formed between a metal and a non-metal.
Chemical formula of sugar is
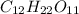 . As carbon, hydrogen and oxygen are all non-metals hence, there will be sharing of electrons between these atoms.
. As carbon, hydrogen and oxygen are all non-metals hence, there will be sharing of electrons between these atoms.
As a result, sugar is a covalent compound and when dissolved in water it does not dissociate into ions. Therefore, a sugar solution is unable to conduct electricity.
Therefore, we can conclude that the statement it is made up of atoms that are held together by covalent bonds, describes sugar.