Answer: Temperature
Step-by-step explanation:
According to the ideal gas equation, which is a combination of Boyle's law, Charle's law , Gay lussac's law and Avogadro's law.
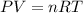
P = Pressure of the gas
V= Volume of the gas
T= Temperature of the gas
R= Gas constant
n= moles of gas

Thus if pressure, volume and the number of moles of a gas are known, temperature is needed to calculate the gas constant.