Answer:
A. H>0, S>0 , G<0
Step-by-step explanation:
Hello,
Based on the noticed change in temperature, one can identify that the enthalpy for this dissolution process is positive as long as the temperature decreases, so Δ
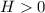 .
.
Now, due to the fact of the bonds breaking down, one establishes that the entropy increases in a positive way, thus Δ
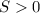 .
.
Finally, by considering that the ammonium nitrate is being dissolved into the water a spontaneous process is carried out, implying that the change in Gibbs free energy turns negative, Δ
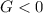 , so one sum up that the answer is A.
, so one sum up that the answer is A.
Best regards.