Answer: hydrochloric acid
Explanation:
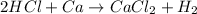
As can be seen from the chemical equation, 2 moles of hydrochloric acid react with 1 mole of calcium.
According to mole concept, 1 mole of every substance weighs equal to its molar mass.
Thus
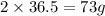 of of hydrochloric acid reacts with 40 g of calcium.
of of hydrochloric acid reacts with 40 g of calcium.
100 g of hydrochloric acid react with=
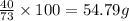 of calcium
of calcium
Thus HCl is the limiting reagent as it limits the formation of product and calcium is the excess reagent as (100-54.79)=45.21 g is left unreacted.