Answer:
32.171087 grams of oxygen will react with 10.47 grams of benzene.
Step-by-step explanation:
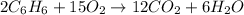
Moles of benzene =
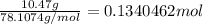
According to reaction ,2 moles of benzene reacts with 15 moles of oxygen
Then 0.1340462 moles of benzene will react with:
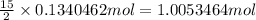 of oxygen
of oxygen
Mass of 1.0053464 moles of oxygen ;
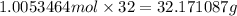
32.171087 grams of oxygen will react with 10.47 grams of benzene.