Answer : The correct option is, (4)
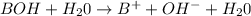
Explanation :
Strong acid : Strong acids are those acids which are completely dissociates into ions in water. It dissociates into hydrogen ion and an anion.
Strong base : Strong base are those base which are completely dissociates into ions in water. It dissociates into hydroxide ion and a cation.
In all the given options, option (4)
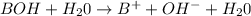 equation shows the complete dissociation of a strong base.
equation shows the complete dissociation of a strong base.
While the other options shows the complete dissociation of an acid.