Answer:
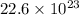 molecules
molecules
Step-by-step explanation:
To calculate the moles, we use the equation:
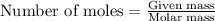
For

Given mass= 165 g
Molar mass of
 = 44 g/mol
= 44 g/mol
Putting values in above equation, we get:
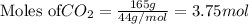
According to avogadro's law, 1 mole of every substance weighs equal to the molecular mass and contains avogadro's number
 of particles.
of particles.
1 mole of
 contains =
contains =
 molecules of
molecules of

Thus 3.75 moles
 contains =
contains =
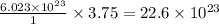 molecules of
molecules of

Thus there will be
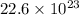 molecules of
molecules of
