Answer: 238.6 J
Step-by-step explanation:
According to the law of conservation of energy, energy can neither be created nor be destroyed. It can only be transformed from one form to another.
Endothermic reactions are those in which heat is absorbed by the system and thus the energy of products is higher than the energy of reactants.
For the given reaction:
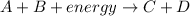
Energy of A = 85.1 J
Energy of B = 87.9 J
Energy on reactant side = Energy of A + Energy of B + Energy absorbed 85.1 + 87.9 + 104.3 = 277.3 J
Energy on reactant side = Energy on product side = 277.3 J
Energy on product side = Energy of C + Energy of D
277.3 J = 38.7 J + Energy of D
Energy of D = 238.6 J
Thus chemical energy product D must contain is 238.6 J