Answer: 8.25 moles of oxygen are produced.
Explanation: The reaction follows:
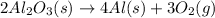
By Stoichiometry,
As, 4 moles of Aluminium is produced by 2 moles of aluminium oxide
11 moles of aluminium will be produced by =
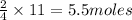
Now, 2 moles of aluminium oxide produces 3 moles of oxygen, so
5.5 moles of Aluminium oxide will produce =
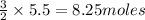
Hence, 8.25 moles of oxygen will be produced.