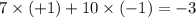Answer: The overall charge on this ion is -3.
Solution:
Protons : These are the subatomic species which carries positive charge.
Electrons: These are the subatomic species which carries negative charge.
Neutrons: These are the subatomic species which doesn't carry any charge.
The net charge on the ion will be = (number protons)
 (charge carried by the protons.) + (total number of electrons)
(charge carried by the protons.) + (total number of electrons)
 (charge carried by the electrons)
(charge carried by the electrons)
Numbers of proton = 7
Numbers of electrons = 10
So, net charge on the nitride ion =