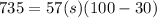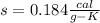Answer:
specific heat capacity of aluminium is 0.184 Calroie/ gram-K.
Step-by-step explanation:
As we know that heat transferred to a given material to change its temperature is given as
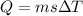
here we know
Q = heat given = 735 calorie
m = mass = 57 gram
initial temperature = 100 degree C
final temperature = 30 degree C
now by above formula we will have