Step-by-step explanation:
When a compound dissociates into two atoms or ions then the process is known as dissociation.
For example,
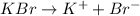
Since, KBr is an ionic compound so it readily dissolves into water (polar solvent) and dissociates into
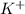 and
and
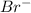 ions and this process is known as dissociation.
ions and this process is known as dissociation.