Answer: C)
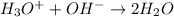
Step-by-step explanation:
 is hydrogen ion,
is hydrogen ion,
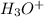 is hydronium ion and
is hydronium ion and
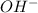 is hydroxide ion.
is hydroxide ion.
The question asks about the neutralization reaction between a hydronium ion and a hydroxide ion.
first equation is taking place between hydrogen ion and water so it is not the right choice.
Second equation is ionization of water into its ions so it is also not the correct choice.
Third equation is taking place between hydronium ion and hydroxide ion and so it is correct.
Fourth equation is taking place between water and ammonia and so it is also not correct.
So, the only correct one is C)
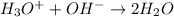 .
.