Answer: Exothermic
Step-by-step explanation: Combustion reaction is a chemical reaction in which hydrocarbon burns in the presence of oxygen to form carbon dioxide, water and energy.
Exothermic reactions are those in which energy is released and endothermic reactions are those in which energy is absorbed.
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution.
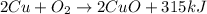
As in the given reaction, the energy is released thus reaction s exothermic.