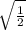Answer:
Ratio of average kinetic energy will be 1:1.
Ratio of root mean square speeds of gases:
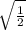
Step-by-step explanation:
Given the mixture of two gases ,at same temperature T.
Gas A = Sulfur oxide =
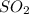
Molar mass of sulfur oxide ,M= 0.064 kg/mol
Gas B = Oxygen gas =

Molar mass of oxygen gas ,M'= 0.032 kg/mo
Average kinetic energy of a molecule of a gas is given as:
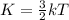
K = Boltzmann constant
T = temperature of the gas
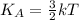 ..(1)
..(1)
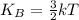 ..(2)
..(2)
Ratio of average kinetic energy will be:
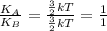
Root mean square speeds of the gas particles:
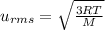
M = molar mass of the gas
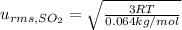 ..(3)
..(3)
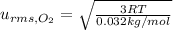 ..(4)
..(4)
Ratio of root mean square speeds of gases:

Ratio of average kinetic energy will be 1:1.
Ratio of root mean square speeds of gases: