Answer:
Time till the 99% of the iodine is out of the system is 5.82 hours.
Step-by-step explanation:
Initial amount of Iodine-134 =
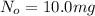
Final amount of iodine-134 left = N =
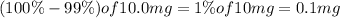
Half life of iodine-134 =
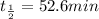
Time till the 99% of the iodine is out of the system = t
Decay constant =
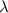
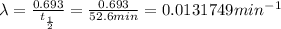
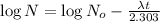

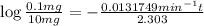
t = 349.60417 min =5.8267 hours
(1 hour = 60 min)
Time till the 99% of the iodine is out of the system is 5.82 hours.